During the EFORT Implant, Patient & Staff Safety Initiative inaugural workshop on 21 January 2020 in Brussels with key decision makers and stakeholders, EFORT presented this new concept and discussed issues around development, introduction and clinical application of implants with a focus on arthroplasty-related topics.
This initiative is strongly consistent with EFORT’s strategic pillars (e.g. harmonisation of guidelines and standards, influencing European health policy) and we believe that EFORT and its stakeholders will take a leading role on this important matter.
During the workshop, three working groups were created in order to discuss the conception of concepts for further action and communication, including patient information, collaborative position papers, proposals for training and education. One of the goals was, to develop recommendations on all three workshop themes, which can then be distributed to stakeholders (i.e. clinicians, manufacturers, regulators, health politics, patients) in order to provide information on important implant-related topics where clinical need has been identified.
Since the workshop took place in January 2020, these three working groups have been actively working on advancing the aims of this initiative:
- Group I: Introduction of innovations.
- Group II: Off-label use, mix & match and clinical consequences of re-certification.
- Group III: Collection and Analysis of Implants Retrieved during Revision.

Group I: Introduction of innovations
Led by Søren Overgaard and Thomas M. Grupp.
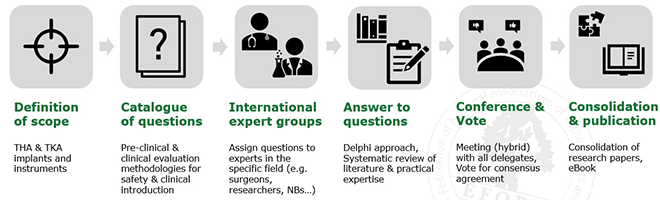
As an outcome from the workshop, a first White Paper was drafted with the title “Introduction of innovations in artificial joint arthroplasty: Recommendations from the EFORT Implant and Patient Safety Initiative” and shared with all workshop participants for review and feedback.
The revised manuscript was then made available to all EFORT National Members in order to also obtain their feedback. In parallel, a survey was conducted to get an overview of the current opinions and mind-sets in relation to implant and patient safety, as well as the clinical introduction of medical devices under the Medical Device Regulation, i.e. the new EU regulation for assessment and registration of medical products.
The second draft of the White Paper as well as the survey results were presented in October 2020 during the first Virtual EFORT Congress (VEC 2020).
The Chairs of this working group have designated a Scientific Committee who have put in place a process for a more intensive and detailed consensus initiative involving EFORT National Members as well as International Expert Delegates.
The Objectives of an “European Consensus on Medical & Scientific Research Requirements for the clinical introduction of orthopaedic joint replacement devices” are:
- To focus on patient safety & performance requirements.
- To maintain innovation and further optimisations of orthopaedic medical products & workflows within the boundaries of MDR 2017/745.
- To establish guidance based on suitable pre-clinical & clinical evaluation methodologies (state of the art 2021).
Consensus has been obtained by a broad dialogue of international orthopaedic surgeons, academic research institutes and experts in biomechanical, medical and scientific research from laboratories and orthopaedic device manufacturers, notified bodies, national member societies, and European Patient Associations. The Consensus process passed the following steps:
Publication of the 1st EFORT European Consensus on “Medical & Scientific Research Requirements for the Clinical Introduction of Artificial Joint Arthroplasty Devices”
The Consensus Book provides:
- 19 topics of relevance addressed by experts (e.g. pre-clinical methods, evaluation of instruments, usability, pre-CE studies).
- 32 Open Access Practical Guidelines with vote on adequate pre-clinical & clinical evaluation methodologies for the clinical introduction.
- Focus on patient safety & performance requirements in arthroplasty.
- Orientation for Surgeons, Research Institutes & Labs, Manufacturers, Notified Bodies, National Institutes & Authorities, Patient Representatives etc.
We thank the following people for their involvement in the 1st EFORT European Consensus on “Medical & Scientific Research Requirements for the Clinical Introduction of Orthopaedic Joint Replacement Devices”.
Scientific Committee
- Ashley Blom | United Kingdom
- Luca Cristofolini | Italy
- Eduardo García-Rey | Spain
- Alexander Giurea | Austria
- Bernd Grimm | Luxemburg
- Marcus Jäger | Germany
- Dennis Janssen | The Netherlands
- Philippe Massin | France
- Francesco Siccardi | Switzerland
International Experts
- Hassan Achakri | Switzerland
- George Babis | Greece
- Francesco Benazzo | Italy
- Dario Bergadano | Switzerland
- Massimiliano Bernardoni | Italy
- Marc Bohner | Switzerland
- Barbara Bordini | Italy
- Joshua Bridgens | United Kingdom
- Vincenzo Carbone | Italy
- Justin Cobb | United Kingdom
- José Cordero-Ampuero | Spain
- Marcel Dreischarf | Germany
- Georg Duda | Germany
- Matthias Fink | Germany
- John Fisher | United Kingdom
- Karlmeinrad Giesinger | Switzerland
- Faris Haddad | United Kingdom
- Alister Hart | United Kingdom
- Petra Heesterbeek | The Netherlands
- Bernardo Innocenti | Belgium
- Volkmar Jansson | Germany
- Christian Kaddick | Germany
- Franz Kainberger | Austria
- Johan Kärrholm | Sweden
- Jan Philipp Kretzer | Germany
- Ricardo Larrainzar-Garijo | Spain
- Christoph Lohmann | Germany
- Anne Lübbeke-Wolff | Switzerland & United Kingdom
- Fabio Mancino | Italy
- Bernard Masson | France
- Richard Mayer | Switzerland
- Claudia Mazza | United Kingdom
- Michael Morlock | Germany
- Caroline Mouton | Luxemburg
- Rob Nelissen | The Netherlands
- Maria Angeles Pérez Ansón | Spain
- Gwendolen Reilly | United Kingdom
- Claude Rieker | Switzerland
- Sabine Rusch | Germany
- Ronja Schierjott | Germany
- Christoph Schilling | Germany
- Arndt-Peter Schulz | Germany
- William Taylor | Switzerland
- Francesco Traina | Italy
- Walter Van der Weegen | The Netherlands
- Marco Viceconti | Italy
- Jan Victor | Belgium
- Henning Windhagen | Germany
- Matthias Woiczinski | Germany
- Ina Wüstefeld | Germany

Group II: Off-label use, mix & match and clinical consequences of re-certification
Led by Luigi Zagra and Keith Tucker.
After a very good initial presentation of the topic during the workshop, it was agreed that there is urgent need to advise clinicians on how to handle different situations, during which – especially in revision cases – products from industry, which might be necessary in order to replace parts of implants (i.e. liners) are not available anymore. If there is only a need for partial exchange, it might be less complicated and dangerous for patients to use other components than the original ones.
This working group has been working on developing a draft on “EFORT-Recommendations for Off-label use and mix & match in hip and knee arthroplasty”. This manuscript has been published in the EFORT Open Reviews.
We thank the following people for their involvement in drafting the EFORT Recommendations for Off-label use, Mix & Match and Mismatch in Hip and Knee Arthroplasty:
- Hassan Achakri | Switzerland
- Neil Betteridge | United Kingdom
- Esther-Patricia Cónsul-Tejero | Germany
- Jean-Alain Epinette | France
- Nils Hailer | Sweden
- Per Kjaersgaard-Andersen | Denmark
- Jan-Philippe Kretzer | Germany
- Jörg Lützner | Germany
- Francesco Marchetti | Italy
- Peter Muenger | Switzerland
- Michaela Münnig | Germany
- Rob Nelissen | The Netherlands
- Philippe Neyret | France
- Luca Orlandini | Switzerland
- Gilles Pasquier | France
- Stefan Ruck | Germany
- Xavier Sarabia | United States
- Raisa Sattar | United Kingdom
- Keith Tucker | United Kingdom
- Cees Verheyen | The Netherlands
- Katharina von Strünsee | Germany
- Dieter Wiek | Germany
- Luigi Zagra | Italy
- Josef Zündorf | Germany

Group III: Collection and Analysis of Implants Retrieved during Revision
Led by Michael Morlock and Enrique Gomez-Barrena.
Failure of a joint replacement procedure is a complex topic. Historically treatment failures were frequently related to implant design and material. Today, arthroplasty registries have helped to identify problematic designs and materials and consecutively remove them from the market step by step.
Contemporary analyses of registry results focus on the surgical process and individual patient characteristics, since these factors in combination with the proper procedure, dominate the clinical success in the patient. Yet problems related to the implant itself still exist and comprise a serious concern in the European society, heralded by occasional media interpretation of problems with new implants and medical devices.
The goal of this working group is to develop a common approach to the collection and analysis of retrieved implants as well as the investigation of a possible relation to the failure of the treatment. A consensus on how to collect and document the analysis of the retrieved implants will also contribute greatly to the process of introducing new implants into the market, which is addressed by group I of this initiative. Finally, the post market surveillance of companies monitoring the safety of their products will also benefit from such a standardized approach. A draft on “EFORT recommendations on storage and documentation of failed implants” is being prepared by the group.
We thank the following people for their involvement in drafting these EFORT Recommendations:
- Atilla Bulent |Turkey
- Dario Bergadano |Switzerland
- Joshua Bridgens | United Kingdom
- Esther-Patricia Cónsul-Tejero | Germany
- José Cordero-Ampuero | Spain
- Antti Eskelinen | Finland
- Li Felländer-Tsai | Sweden
- Michael Georg | Germany
- Alister Hart | United Kingdom
- Theodore P. Kormas | Greece
- Justyna Kozik-Jaromin | Germany
- Katre Maasalu | Estonia
- Jan Noyez | Belgium
- Luca Orlandini | Switzerland
- Rihard Trebše | Slovenia
- Gijs Van Hellemondt | The Netherlands
- Dieter Wirtz | Germany
Orthopaedic and Traumatology innovations have improved care for musculoskeletal disorders and injuries, with new implants, techniques, and research improving patient outcomes. The 1st EFORT European Consensus on “Medical & Scientific Research Requirements for the Clinical Introduction of Artificial Joint Arthroplasty Devices” aims to maintain innovation and optimize orthopaedic products while respecting MDR boundaries.
MORE INFORMATION | PDF Publication | 247 pages | 8 Mb
IPSSI-sessions at the EFORT Congress 2023 in Vienna:
- Metals In Implants: Implications For Patients And Novel Alternatives
Wednesday 24 May | 02:45 – 03:45 PM CET
IPSSI-sessions at the EFORT Congress 2022 in Lisbon:
- Medical Device Regulation (MDR) In Active Mode – Current Effects On Orthopaedics And Traumatology
Wednesday 22 June | 04:15 – 05:15 PM CET - Challenges For Health Systems And Staff During War
Wednesday 22 June | 02:45 – 03:45 PM CET
IPSSI-sessions at the VEC 2021:
- EFORT Implant Safety Initiative: Introduction Of Innovations In Arthroplasty And Failure Analysis
Wednesday 30 June | Channel 1 | 09:30-10:30 AM CET - EFORT Implant Safety Initiative: Off-Label Use And Mix & Match
Wednesday 30 June | Channel 1 | 11:00-12:00 AM CET - Implant & Patient Safety Initiative (IPSI) & Consensus Conference
Wednesday 30 June | 06:00-06:30 PM CET
For further information or questions about the
EFORT Implant, Patient & Staff Safety Initiative (IPSSI), please contact: